[ad_1]
The body is pretty good at repairing itself, but some parts of our anatomy struggle to bounce back after an injury.
One such material is cartilage – the spongy yet firm connective tissue that keeps our bones from rubbing and jarring against each other. Over time, the translucent or ‘hyaline’ components of cartilage can become heavily degraded, resulting in painful conditions like osteoarthritis and chondromalacia.
Scientists have been working on a way to regenerate hyaline cartilage for years, and now a team led by Northwestern University in the US has achieved a breakthrough. They have developed a biomaterial that, injected into damaged cartilage in living sheep, acted as a scaffold that promoted cartilage regrowth in active joints.
“Cartilage is a critical component in our joints,” says chemist Samuel Stupp of Northwestern University.
“When cartilage becomes damaged or breaks down over time, it can have a great impact on people’s overall health and mobility. The problem is that, in adult humans, cartilage does not have an inherent ability to heal. Our new therapy can induce repair in a tissue that does not naturally regenerate. We think our treatment could help address a serious, unmet clinical need.”
Cartilage deterioration is a massive health issue. A 2011 survey of 18 countries found over 1.3 million total knee replacement surgeries being conducted every year, with cartilage problems being a major factor.
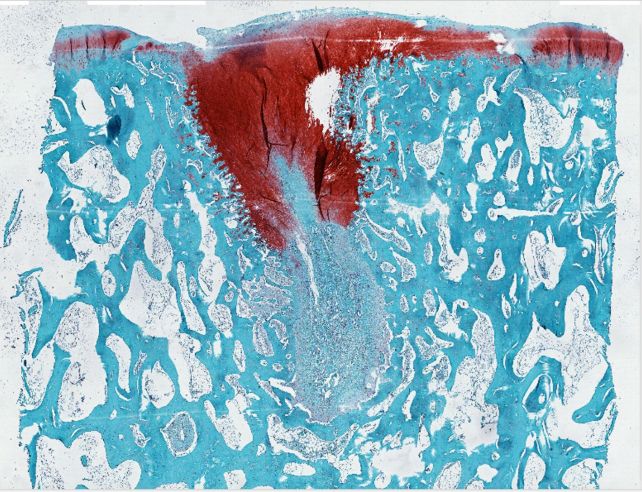
And, while there are other interventions that can help, such as creating microfractures to induce cartilage repair, these often result in the growth of the wrong kind of cartilage – tough, fibrous fibrocartilage – like cartilaginous scar tissue – instead of the smooth, bouncy hyaline cartilage that covers joints.
One possible treatment scientists have been refining is the use of scaffolds in cartilage defects that support the growth of hyaline cartilage. This option has shown promise in small mammals, such as mice, but fails in bigger mammals, likely because of the greater stresses and forces at work on the joints of larger animals.
Stupp and his colleagues have developed a material that seems to resolve this glaring problem. It’s a biomaterial, made from two components.
The first is a peptide molecule that binds to a protein called transforming growth factor beta-1, which plays a crucial role in the growth and division of cells, especially in the skeleton, where it regulates the growth of bone and cartilage.
The second component is hyaluronic acid, which you might know from your skincare products – it helps your skin stay soft by retaining moisture. It can also be found in the joints and cartilage, where it acts as a lubricant, and plays a role in wound healing.
The researchers found in test tube experiments this hybrid material supported chondrogenic differentiation – that is, the proliferation of cartilage cells, known as chondrocytes. It formed bundles of filaments, a natural structure found in musculoskeletal tissues.
A lot of research avenues have reached the same point in vitro. The real test would be how it stood up to a living animal that can be extrapolated to a human. Sheep experience similar mechanical loading to humans, so sheep is where the testing took place.
The researchers developed their material into a slurry that was injected into cartilage defects in rear knee joints in sheep. The researchers introduced small holes into both rear knees on each of the sheep in their study; one knee was injected with the slurry, and the other left untreated as a control.
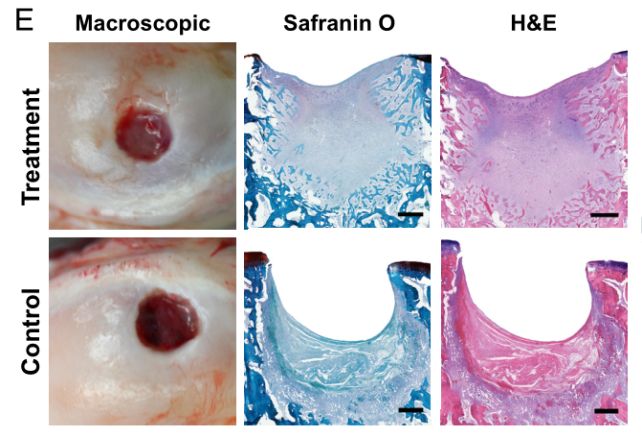
When the slurry came into contact with the calcium in the sheeps’ bones, it hardened into a rubbery matrix, and chondrocytes started forming, growing to fill the introduced hole as the scaffold degraded. And it was the right kind of cartilage: hyaline. Within weeks, the treated defects showed a remarkable improvement, especially compared to the controls.
A similar treatment might be even more effective in humans. You can’t tell a sheep that they need to rest and keep their weight off an injured leg, whereas patients receiving treatment for cartilage injuries are often immobilized after surgery.
Further research and development is required, of course, and then the process will have to undergo human clinical trials. But the findings show promise that a treatment will one day become available that will help reduce the need for more invasive, and less effective, options.
“By regenerating hyaline cartilage,” Stupp says, “our approach should be more resistant to wear and tear, fixing the problem of poor mobility and joint pain for the long term while also avoiding the need for joint reconstruction with large pieces of hardware.”
The research has been published in the Proceedings of the National Academy of Sciences.
[ad_2]
